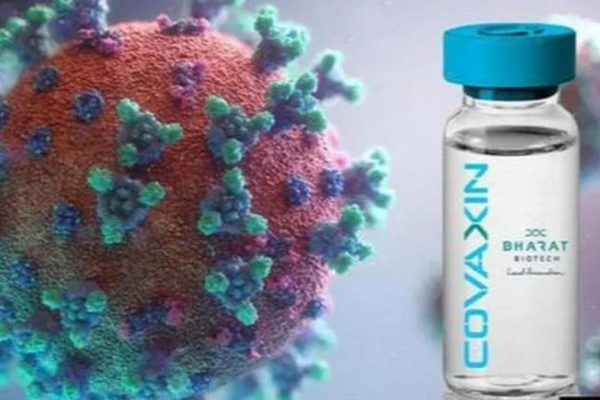
US FDA approves Bharat Biotech’s Covaxin for clinical trails
The US Food and Drug Administration has lifted the clinical hold on Bharat Biotech's Covaxin.
Stay updated with the latest news and articles about Bharat BioTech’s Covaxin.

The US Food and Drug Administration has lifted the clinical hold on Bharat Biotech's Covaxin.

From November 8, the United States will allow passengers who have been vaccinated with Covaxin to enter the country.

Bharat Biotech recently stated it has submitted all of the information bearings on its COVID-19 vaccine Covaxin to the World health organization (WHO) for Emergency Use Listing (EUL) and is anticipating feedback.

Bharat Biotech owned COVID-19 vaccine, Covaxin accrued Good Manufacturing Practice certification from the Hungarian administration, this Thursday.


The COVAXIN is an inactive vaccine that is developed by chemically treating the novel coronavirus samples to make it incapable of reproduction. That process leave the viral proteins intact, also including the spike protein of the coronavirus which it uses to enter the human cells.



Ministry of Health issued a statement denying media reports of not placing any fresh order for Covid vaccines since March 2021. While the ministry declares that the allegations are a pile of complete rubbish.






Showing 15 of 16 articles
