The migraine therapeutics market is experiencing strong momentum as rising global prevalence, better diagnostic recognition, and expanding patient awareness steadily grow the treated population. Adoption of targeted therapies such as CGRP inhibitors and gepants continues to accelerate, driven by their superior tolerability and preventive benefits compared to older acute treatments. At the same time, telehealth expansion and wider access to specialty care are improving treatment initiation and follow-up, supporting a sustained shift toward guideline-based management. With a robust pipeline of biologics, oral agents, and device-based innovations, the competitive landscape is expected to broaden further, reinforcing long-term market growth potential.
New York, USA, Dec. 02, 2025 (GLOBE NEWSWIRE) — Migraine Clinical Trial Pipeline Expands as 30+ Companies Driving Innovation in Oncology Therapeutics Space | DelveInsight
The migraine therapeutics market is experiencing strong momentum as rising global prevalence, better diagnostic recognition, and expanding patient awareness steadily grow the treated population. Adoption of targeted therapies such as CGRP inhibitors and gepants continues to accelerate, driven by their superior tolerability and preventive benefits compared to older acute treatments. At the same time, telehealth expansion and wider access to specialty care are improving treatment initiation and follow-up, supporting a sustained shift toward guideline-based management. With a robust pipeline of biologics, oral agents, and device-based innovations, the competitive landscape is expected to broaden further, reinforcing long-term market growth potential.
DelveInsight’s ‘Migraine Pipeline Insight 2025‘ report provides comprehensive global coverage of pipeline therapies for migraine across various stages of clinical development. The report offers an in-depth analysis of key trends, emerging therapies, and competitive landscape dynamics, highlighting the strategies of major pharmaceutical companies to advance the pipeline and capitalize on future growth opportunities. In addition, it includes critical insights into clinical trial benchmarking, partnering and licensing activities, and regulatory pathways involving the FDA and EMA, enabling stakeholders to make informed decisions and optimize development strategies within the migraine domain.
Migraine Clinical Trial Analysis Summary
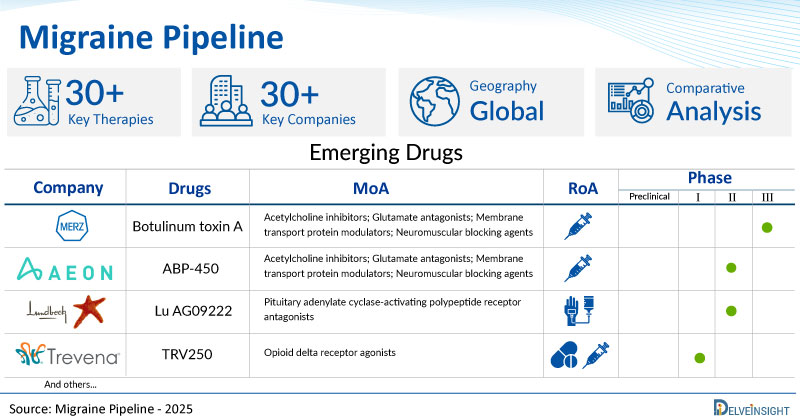
- DelveInsight’s migraine pipeline report depicts a robust space with 30+ active players working to develop 30+ pipeline migraine drugs.
- Key migraine companies such as Merz Therapeutics, H. Lundbeck A/S, Biohaven, Ipsen, Kallyope Inc., Pulmatrix, AEON Biopharma, Trevena, Nuvie Bio, Miist Therapeutics, Galt Pharmaceuticals, and others are evaluating new migraine drugs to improve the treatment landscape.
- Promising pipeline migraine therapies, such as Botulinum toxin A, Lu AG09222, BHV-2100, IPN10200, K 645, PUR3100, ABP-450, TRV250, NVI-100, MST02, GA 2601, and others, are in different phases of migraine clinical trials.
- Approximately 3 migraine drugs are in the late stage of development, whereas 10 drugs are in the mid and early stages of development.
- Notable MoAs in migraine clinical trials include Acetylcholine inhibitors; Glutamate antagonists; Membrane transport protein modulators; Neuromuscular blocking agents, Pituitary adenylate cyclase-activating polypeptide receptor antagonists, Opioid delta receptor agonists, and others.
Request a sample and discover the recent advances in migraine drugs @ Migraine Pipeline Report

What is Migraine?
Migraine is a neurological disorder that causes intense pain and affects millions globally. It is the most prevalent neurological condition, significantly impacting brain function and influencing behaviors related to recurrent migraine episodes. The burden of migraine involves processes occurring before, during, and after an attack. Understanding migraine includes recognizing that normally non-painful stimuli, such as light, can intensify pain, and that migraine headaches can lead to widespread skin sensitivity. Advanced imaging studies have revealed changes in brain structure and function, offering more profound insights into the disease mechanisms. Migraines affect people worldwide and are often misdiagnosed as other types of headaches, such as tension-type headaches. Many sufferers may not receive an accurate diagnosis, effective treatment, or adequate support from family, friends, or colleagues. Treatment typically involves acute or abortive medications, while a smaller portion of patients use preventive therapies. Migraines remain a significant cause of global disability and suffering. Risk factors include both non-modifiable elements, such as genetics, gender, and age.
Find out more about migraine drugs @ Migraine Treatment
A snapshot of the Pipeline Migraine Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| Botulinum toxin A | Merz Therapeutics | III | Acetylcholine inhibitors; Glutamate antagonists; Membrane transport protein modulators; Neuromuscular blocking agents | Intramuscular |
| ABP-450 | AEON Biopharma, Inc. | II | Acetylcholine inhibitors; Glutamate antagonists; Membrane transport protein modulators; Neuromuscular blocking agents | Intramuscular |
| Lu AG09222 | H. Lundbeck A/S | II | Pituitary adenylate cyclase-activating polypeptide receptor antagonists | Intravenous |
| TRV250 | Trevena | I | Opioid delta receptor agonists | Oral/ Subcutaneous |
| MST02 | Miist Therapeutics | Preclinical | Undefined mechanism | Inhalation |
Learn more about the emerging migraine therapies @ Migraine Clinical Trials
Recent Developments in Migraine Treatment Space
- In November 2025, AEON Biopharma, Inc. announced the first closing of its previously announced private placement (“PIPE”). Additionally, the Company announced that the US Food and Drug Administration (the “FDA”) has proposed a new date of January 21, 2026 for AEON’s Biosimilar Biological product Development (BPD) Type 2a meeting. The meeting had previously been scheduled for November 19, 2025.
- In October 2025, AEON Biopharma, Inc. announced that the US Food and Drug Administration (FDA) has scheduled a Biosimilar Biological Product Development (BPD) Type 2a meeting for ABP-450 on November 19, 2025, in line with prior guidance.
- In February 2025, Miist Therapeutics, announced USD 7M in seed funding from investors including Refactor Capital, 1517 Fund, Freeflow Ventures, Entrepreneur First, and California Innovation Fund, among others. Miist has clinically validated its approach and is currently advancing two assets: MST-01 for the treatment of smoking addiction and MST-02 for the treatment of migraine. With this funding, the company is now positioned to accelerate its assets one step closer to the nearly 100 million patients who need them globally.
- In January 2025, Nuvie Bio, announced completion of a first-in-human trial with NVI-100, the company’s lead investigational drug for the acute treatment of migraine.
- In September 2024, Biohaven Ltd. announced that it has initiated a pivotal Phase II study of the potential first-in-class, orally administered TRPM3 antagonist, BHV-2100, in the acute treatment of migraine.
- In May 2024, Pulmatrix announced publication of, “Safety, tolerability, and pharmacokinetics of a single orally inhaled dose of PUR3100, a dry powder formulation of dihydroergotamine versus intravenous dihydroergotamine: A Phase I randomized, double-blind study in healthy adults” in the peer-reviewed publication Headache: The Journal of Head and Face Pain.
- In May 2024, AEON Biopharma, Inc. announced that the preliminary top-line results from its planned interim analysis of the Phase II trial with ABP-450 in the preventive treatment for chronic migraine did not meet the primary endpoint.
Scope of the Migraine Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Intra-articular, Intraocular, Intrathecal, Intravenous, Ophthalmic, Oral, Parenteral, Subcutaneous, Topical, Transdermal
- Therapeutics Assessment By Molecule Type: Oligonucleotide, Peptide, Small molecule
- Therapeutics Assessment By Mechanism of Action: Serotonin 1B receptor agonists, Serotonin 1D receptor agonists, Acetylcholine inhibitors, Glutamate antagonists, Membrane transport protein modulators, Neuromuscular blocking agents, Pituitary adenylate cyclase-activating polypeptide receptor antagonists, Opioid delta receptor agonists, 5-HT2C serotonin receptor antagonists, Serotonin 1F receptor partial agonists, Serotonin 2B receptor antagonists, Serotonin 5-HT2A receptor antagonists, Serotonin 7 receptor agonists, Serotonin-1D-receptor partial agonists
- Key Migraine Companies: Merz Therapeutics, H. Lundbeck A/S, Biohaven, Ipsen, Kallyope Inc., Pulmatrix, AEON Biopharma, Trevena, Nuvie Bio, Miist Therapeutics, Galt Pharmaceuticals, and others.
- Key Migraine Pipeline Therapies: Acetylcholine inhibitors; Glutamate antagonists; Membrane transport protein modulators; Neuromuscular blocking agents, Pituitary adenylate cyclase-activating polypeptide receptor antagonists, Opioid delta receptor agonists, and others.
Dive deep into rich insights for new migraine treatments, visit @ Migraine Drugs
Table of Contents
| 1. | Migraine Pipeline Report Introduction |
| 2. | Migraine Pipeline Report Executive Summary |
| 3. | Migraine Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Migraine Clinical Trial Therapeutics |
| 6. | Migraine Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Migraine Pipeline: Late-Stage Products (Phase III) |
| 8. | Migraine Pipeline: Mid-Stage Products (Phase II) |
| 9. | Migraine Pipeline: Early-Stage Products (Phase I) |
| 10. | Migraine Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Migraine Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Migraine Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the migraine pipeline therapeutics, reach out @ Migraine Therapeutics
Related Reports
Migraine Epidemiology Forecast
Migraine Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted migraine epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Migraine Market
Migraine Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key migraine companies, including Lundbeck Seattle BioPharmaceutical, Eli Lilly and Company, Amgen, Novartis, Teva Pharmaceuticals, Allergan, Biohaven Pharmaceuticals, Dr. Reddy’s Labs, AbbVie, Impel NeuroPharma, Zosano Pharma, Axsome Therapeutics, Aeon Biopharma Inc., Ionis Pharmaceuticals, Inc., Charleston Laboratories, among others.
Cluster Headache Market
Cluster Headache Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key cluster headache companies, including Novartis AG, Zosano Pharma, Eli Lily and Company, AstraZeneca Plc., Autonomic Technologies, Inc., ElectroCore Medical LLC, GlaxoSmithKline Plc, Winston Pharmaceuticals Inc., Lundbeck Seattle BioPharmaceutical, among others.
Cluster Headache Clinical Trial Analysis Pipeline
Cluster Headache Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key cluster headache companies, including Novartis AG, Zosano Pharma, Eli Lily and Company, AstraZeneca Plc., Autonomic Technologies, Inc., ElectroCore Medical LLC, GlaxoSmithKline Plc, Winston Pharmaceuticals Inc., Lundbeck Seattle BioPharmaceutical, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Pipeline Assessment
Healthcare Licensing Services
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn
CONTACT: Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com

Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. Business Upturn takes no editorial responsibility for the same.